Adverse Drug Events – Screening & Diagnosis
Other, Toxicology
Context
- Adverse drug events are unintended and harmful events related to medication use or misuse.
- Adverse drug events (ADE) cause or contribute to 1 in 9 emergency department presentations in Canada, and are associated with poor patient outcomes.1
- Emergency physicians do not recognize 40-50% of patients presenting with ADEs2 and the ADE either worsens, or recurs when continues or restarts the culprit medication.
- Emergency departments have insufficient clinical pharmacists to complete medication reviews in all incoming patients to identify adverse drug events.
- Many patients have presentations that are clearly not related to medication use (e.g., acute appendicitis) and do not require a medication review.
Diagnostic Process
This simple and validated clinical decision rule can identify 90% of patients with adverse drug events. Those patients identified as “high-risk” by the rule should have a detailed examination of their medications, to rule out adverse drug events.
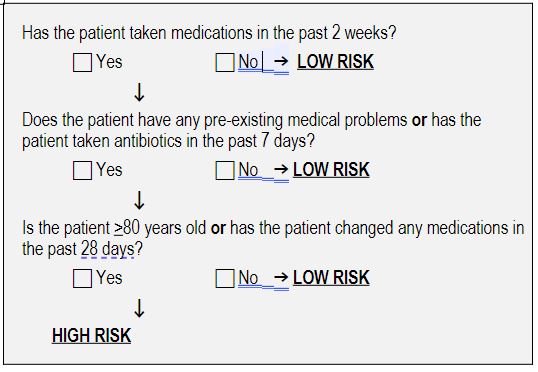
Acad Emerg Med. 2018 Sep; 25(9): 1015–1026. Published online 2018 Sep 11. doi: 10.1111/acem.13407
- a change includes stopping, adding, replacing or changing the dose
Quality Of Evidence?

High
We are highly confident that the true effect lies close to that of the estimate of the effect. There is a wide range of studies included in the analyses with no major limitations, there is little variation between studies, and the summary estimate has a narrow confidence interval.
Moderate
We consider that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. There are only a few studies and some have limitations but not major flaws, there are some variations between studies, or the confidence interval of the summary estimate is wide.
Low
When the true effect may be substantially different from the estimate of the effect. The studies have major flaws, there is important variations between studies, of the confidence interval of the summary estimate is very wide.
Justification
- The ADE rule above has been derived and validated prospectively in four centres in British Columbia and Ontario, including in one community hospital.3 4
- When implemented in three hospitals in the BC Lower Mainland, pharmacist-led medication review resulted in a 10% reduction in median length of hospital stay.5
Related Information
Reference List
-
-
Hohl CM, Badke K, Wickham ME, et al. Clinical decision rules to Improve the detection of adverse drug events in the Emergency Department: A Validation Study. submitted
-
-
Relevant Resources
RELEVANT RESEARCH IN BC
Preventing Adverse Drug EventsRESOURCE AUTHOR(S)

DISCLAIMER
The purpose of this document is to provide health care professionals with key facts and recommendations for the diagnosis and treatment of patients in the emergency department. This summary was produced by Emergency Care BC (formerly the BC Emergency Medicine Network) and uses the best available knowledge at the time of publication. However, healthcare professionals should continue to use their own judgment and take into consideration context, resources and other relevant factors. Emergency Care BC is not liable for any damages, claims, liabilities, costs or obligations arising from the use of this document including loss or damages arising from any claims made by a third party. Emergency Care BC also assumes no responsibility or liability for changes made to this document without its consent.
Last Updated Oct 05, 2023
Visit our website at https://emergencycarebc.ca
COMMENTS (0)
Add public comment…


POST COMMENT
We welcome your contribution! If you are a member, log in here. If not, you can still submit a comment but we just need some information.