Baclofen Toxicity
Toxicology
Background
- Baclofen is a muscle relaxant & antispasmodic commonly used as an adjust therapy for patients with chronic pain and/or spasticity associated with spinal-cord disease.
- It primarily targets receptors in the spinal cord resulting in muscle relaxation. At higher doses it loses this specificity and distributes to the brain causing sedative effects.
- Baclofen toxicity can be life-threatening and is often associated with depressed consciousness, coma, and seizures.
- Patients at greatest risk for Baclofen toxicity are those with acute oral ingestions >200mg; have underlying or new renal impairment; or use intrathecal pumps.
Context
Pathophysiology
- Baclofen is a GABA agonist that binds to GABA-B receptors in the brain and spinal cord.
- At therapeutic concentrations, Baclofen targets the spinal cord to cause muscle relaxation.
- At toxic concentrations, Baclofen loses specificity for the spinal cord and begins to exert greater effect on the brain.
Toxicokinetic
- High bioavailability with rapid absorption and time to peak effect <2hrs.
- Half-life is ~2-6hrs in healthy patients but can be markedly prolonged in the context of overdose or renal impairment.
- Largely renally excreted with the remainder being hepatically metabolized.
- Intrathecal administration has a slower onset and time to peak effect.
History and Physical
- Symptoms and severity can vary widely.
- Mild toxicity usually presents with nonspecific CNS depression (lethargy, confusion, headaches, and nausea) which can progress to myoclonus and hypotonia.
- Moderate-to-severe toxicity is associated with severe CNS depression, respiratory failure, nonconvulsive status, complete flaccidity, and coma with loss brainstem reflexes mimicking brain death.
- Autonomic abnormalities include hypothermia, hypotension, and bradycardia; but paradoxical hypertension and tachycardia have also been reported.
- Cardiac conduction abnormalities include heart block, prolonged QT interval, premature atrial contractions, or SVT.
Investigations
- Measuring plasma concentration is possible, but not routine and does not change management.
- EEG can assist with diagnosis and identify complications (i.e., nonconvulsive status epilepticus).
Management
- Discuss case with poison center.
- There is no specific antidote for baclofen toxicity.
- Supportive care includes:
- Activated charcoal 1g/kg PO/NG can be considered if the timeline is appropriate and airway is protected, but its efficacy in adults is unclear.
- CNS and/or respiratory depression may require securing airway.
- Treat hypotension with IV fluids. Reserve vasopressors for refractory cases.
- Treat bradycardia with atropine if symptomatic.
- Treat seizures with benzodiazepines.
- Dialysis
- HD has been shown to dramatically reduce drug half-life in patients with renal impairment while evidence demonstrating statistically significant reductions in drug half-life in patients with normal renal function is mixed.
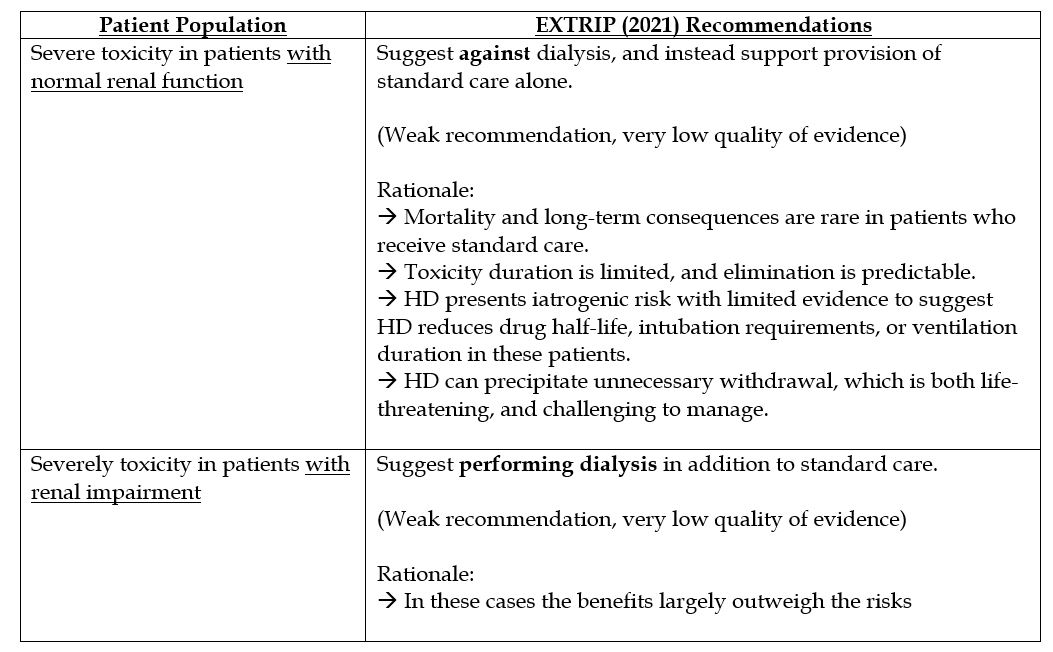
- In cases of rapid clearance (hemodialysis) or abrupt cessation (i.e., stopping an intrathecal pump), clinicians should be prepared to manage Baclofen withdrawal as this is also life threatening.
Disposition
- Admit symptomatic ingestions and consult ICU for cases with airway compromise, hemodynamic instability, or significant CNS manifestations.
- For oral ingestions, baclofen should be held, but restarted when symptoms have resolved to avoid life-threatening withdrawals.
- For overdoses related to an intrathecal pump malfunction, the pump should be stopped. Re-initiation should be planned within 48 hours of stoppage to prevent withdrawal.
Prognosis
- Outcomes are good if patients are stabilized early.
- Most patients will demonstrate improvement of their mental status within 24 – 48 hours.
- Large overdoses (up to 1 to 2g) are more likely to be fatal.
- Overdoses stemming from intrathecal pump malfunction tend to have poorer outcomes.
Related Information
Reference List
Ghannoum M, Berling I, Lavergne V, et al. Recommendations from the EXTRIP workgroup on extracorporeal treatment for baclofen poisoning. Kidney Int. 2021;100(4):720-736. doi:10.1016/j.kint.2021.07.014
Romito JW, Turner ER, Rosener JA, et al. Baclofen therapeutics, toxicity, and withdrawal: A narrative review. SAGE Open Med. 2021;9:20503121211022197. Published 2021 Jun 3. doi:10.1177/20503121211022197
Leung NY, Whyte IM, Isbister GK. Baclofen overdose: defining the spectrum of toxicity. Emerg Med Australas. 2006;18(1):77-82. doi:10.1111/j.1742-6723.2006.00805.x
Dease NM, Kershner EK, Wills BK. Baclofen Toxicity. [Updated 2023 Mar 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK580550/
Meulendijks D, Khan S, Koks CH, Huitema AD, Schellens JH, Beijnen JH. Baclofen overdose treated with continuous venovenous hemofiltration. Eur J Clin Pharmacol. 2015;71(3):357-361. doi:10.1007/s00228-014-1802-y
Wolf E, Kothari NR, Roberts JK, Sparks MA. Baclofen Toxicity in Kidney Disease. Am J Kidney Dis. 2018;71(2):275-280. doi:10.1053/j.ajkd.2017.07.005
RESOURCE AUTHOR(S)

DISCLAIMER
The purpose of this document is to provide health care professionals with key facts and recommendations for the diagnosis and treatment of patients in the emergency department. This summary was produced by Emergency Care BC (formerly the BC Emergency Medicine Network) and uses the best available knowledge at the time of publication. However, healthcare professionals should continue to use their own judgment and take into consideration context, resources and other relevant factors. Emergency Care BC is not liable for any damages, claims, liabilities, costs or obligations arising from the use of this document including loss or damages arising from any claims made by a third party. Emergency Care BC also assumes no responsibility or liability for changes made to this document without its consent.
Last Updated Feb 03, 2024
Visit our website at https://emergencycarebc.ca
COMMENTS (0)
Add public comment…


POST COMMENT
We welcome your contribution! If you are a member, log in here. If not, you can still submit a comment but we just need some information.