Tranexamic Acid (TXA) in the Emergency Department
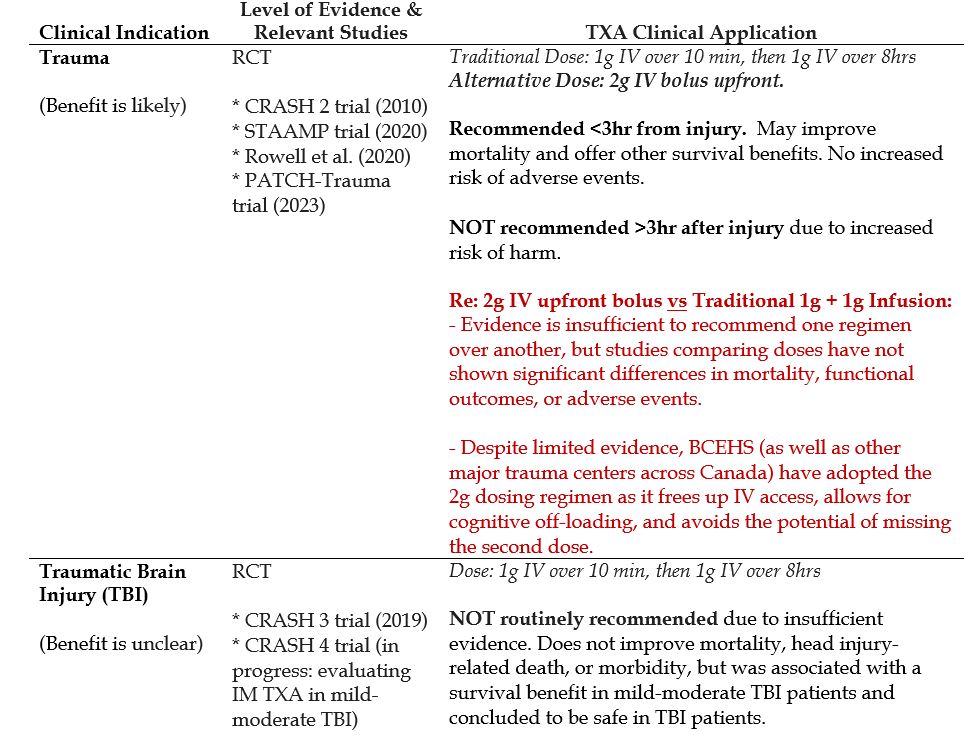
Trauma
Context
- TXA is an antifibrinolytic agent used across multiple clinical settings for its ability to provide hemostatic control and improve clinical outcomes in patients that are bleeding.
- Evidence suggests a modest mortality benefit when used for trauma resuscitation, but it’s utility and efficacy in other clinical scenarios is less clear and requires clinical judgement.
- It does not seem to offer mortality benefit to patients with spontaneous intracranial, or subarachnoid, or gastrointestinal bleeding.
- Optimal dosing in emergency settings is unclear.
- Traditional dosing (based on the landmark CRASH 2 trial) is 1g IV over 10min, followed by 1g IV over the next 8 hrs.
- Emerging evidence suggests that an alternative dose: 2g IV bolus upfront (instead of the traditional 1g + 1g) might be more convenient for providers without significant differences in mortality, functional outcomes, or adverse events.
Pharmacology
- TXA is a synthetic lysine analog that binds to plasminogen which prevents its conversion into plasmin. This results in inhibited fibrinolysis and reduced clot degradation.
- Available PO, IV, IM, topically, or via nebulizer.
- IM and IV administration share similar bioavailability and achieve therapeutic levels < 15 min.
ED Applications
- Trauma
- Traumatic Brain Injury (TBI)
- Intracranial Hemorrhage (ICH)
- Subarachnoid Hemorrhage (SAH)
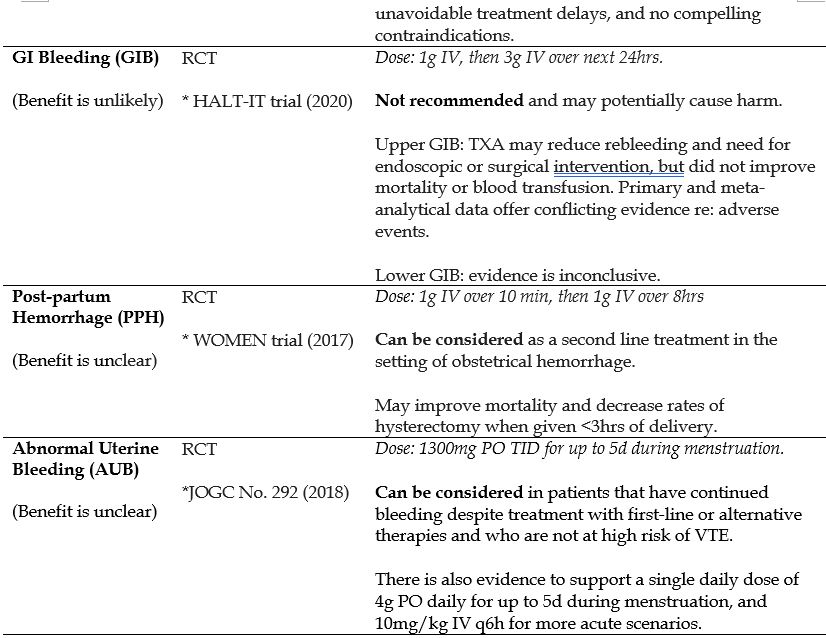
- Gastrointestinal Bleeding (GIB)
- Post-partum Hemorrhage (PPH)
- Abnormal Uterine Bleeding
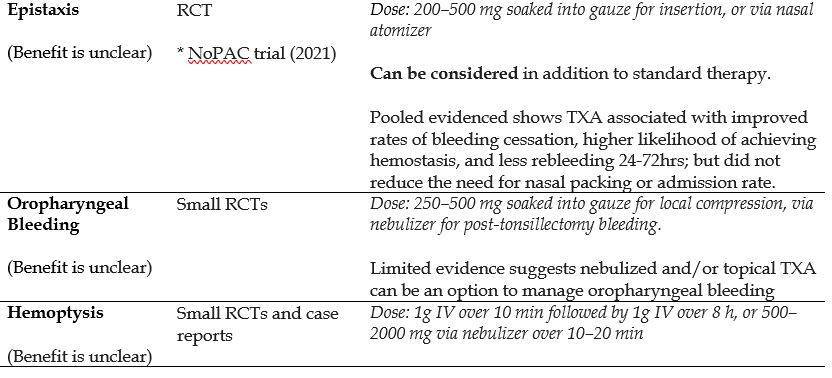
- Epistaxis
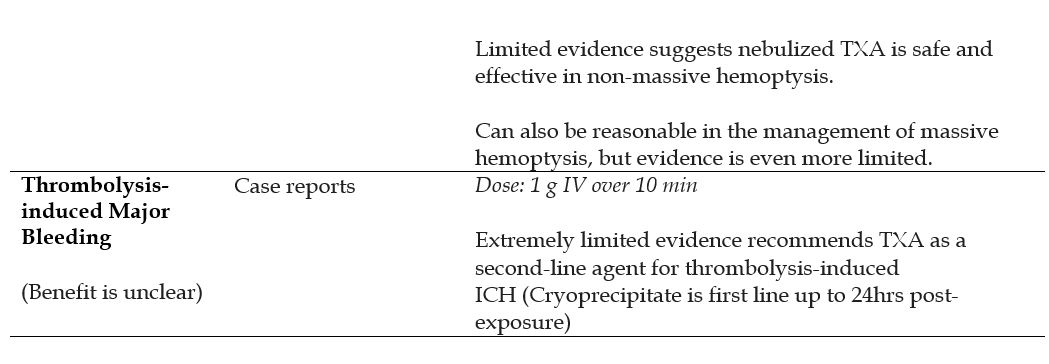
- Hemoptysis
- Thrombolytic-induced Major Bleeding
Recommended Treatment

Related Information
Reference List
Wang K, Santiago R. Tranexamic acid – A narrative review for the emergency medicine clinician [published correction appears in Am J Emerg Med. 2022 May 23;:]. Am J Emerg Med. 2022;56:33-44. doi:10.1016/j.ajem.2022.03.027
CRASH-2 trial collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23-32. doi:10.1016/S0140-6736(10)60835-5
Guyette FX, Brown JB, Zenati MS, et al. Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial [published correction appears in JAMA Surg. 2021 Jan 1;156(1):105]. JAMA Surg. Published online October 5, 2020. doi:10.1001/jamasurg.2020.4350
Mitra B, Bernard S, Gantner D, et al. Protocol for a multicentre prehospital randomised controlled trial investigating tranexamic acid in severe trauma: the PATCH-Trauma trial. BMJ Open. 2021;11(3):e046522. Published 2021 Mar 15. doi:10.1136/bmjopen-2020-046522
CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial [published correction appears in Lancet. 2019 Nov 9;394(10210):1712]. Lancet. 2019;394(10210):1713-1723. doi:10.1016/S0140-6736(19)32233-0
Rowell SE, Meier EN, McKnight B, et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury [published correction appears in JAMA. 2020 Oct 27;324(16):1683]. JAMA. 2020;324(10):961-974. doi:10.1001/jama.2020.8958
Sprigg, K. Flaherty, J.P. Appleton, R.A.,S. Salman, D. Bereczki, M. Beridze, et al.Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial Lancet. 2018;391(10135):2107-2115. doi:10.1016/S0140-6736(18)31033-X
Post R, Germans MR, Tjerkstra MA, et al. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. 2021;397(10269):112-118. doi:10.1016/S0140-6736(20)32518-6
HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10241):1927-1936. doi:10.1016/S0140-6736(20)30848-5
Reuben A, Appelboam A, Stevens KN, et al. The Use of Tranexamic Acid to Reduce the Need for Nasal Packing in Epistaxis (NoPAC): Randomized Controlled Trial. Ann Emerg Med. 2021;77(6):631-640. doi:10.1016/j.annemergmed.2020.12.013
WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet. 2017 May 27;389(10084):2104]. Lancet. 2017;389(10084):2105-2116. doi:10.1016/S0140-6736(17)30638-4
Singh S, Best C, Dunn S, Leyland N, Wolfman WL. No. 292-Abnormal Uterine Bleeding in Pre-Menopausal Women. J Obstet Gynaecol Can. 2018;40(5):e391-e415. doi:10.1016/j.jogc.2018.03.007
RESOURCE AUTHOR(S)

DISCLAIMER
The purpose of this document is to provide health care professionals with key facts and recommendations for the diagnosis and treatment of patients in the emergency department. This summary was produced by Emergency Care BC (formerly the BC Emergency Medicine Network) and uses the best available knowledge at the time of publication. However, healthcare professionals should continue to use their own judgment and take into consideration context, resources and other relevant factors. Emergency Care BC is not liable for any damages, claims, liabilities, costs or obligations arising from the use of this document including loss or damages arising from any claims made by a third party. Emergency Care BC also assumes no responsibility or liability for changes made to this document without its consent.
Last Updated Feb 03, 2024
Visit our website at https://emergencycarebc.ca
COMMENTS (1)
Add public comment…


POST COMMENT
We welcome your contribution! If you are a member, log in here. If not, you can still submit a comment but we just need some information.