Complicated UTI/Urosepsis Management
Cardinal Presentations / Presenting Problems, Infections, Urological
Context
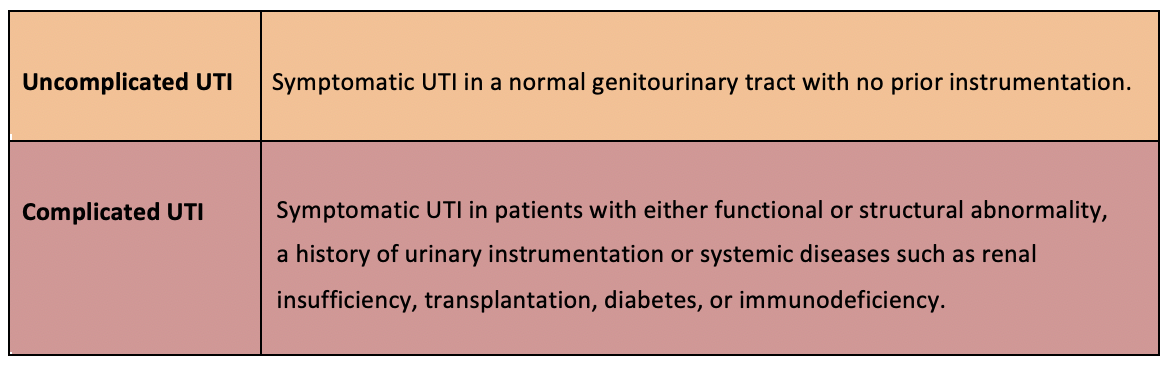
Complicated UTI
- UTI with systemic symptoms: fever, chills, fatigue; flank pain, CVA tenderness.
- In men, consider prostatitis, especially if recurrent cystitis symptoms, or pelvic/perineal pain.
- Digital Rectal Examination warranted
- Please refer to UTI Special Circumstances – UTI in Men
- In men, consider prostatitis, especially if recurrent cystitis symptoms, or pelvic/perineal pain.
- May develop sepsis or renal abscess.
- Risk factors include diabetes, urinary tract obstruction.
- Perform both urinalysis (Urine dipstick and/or microscopy) and urine culture and sensitivity (urine gram stain can also be helpful).
- Pyuria and bacteriuria support diagnosis (+/- hematuria)
- See UTI Diagnostic Approach
- Other investigations include:
- Blood cultures if sepsis suspected
- Pregnancy testing if appropriate
- Imaging performed if:
- Severe illness (e. sepsis)
- No improvement with antibiotic therapy
- Suspicious for urinary tract obstruction, renal abscess
- CT is first choice (U/S if concern for radiation exposure)
- Microbiology:
- E. coli most common, then:
- Klebsiella, Proteus
- Pseudomonas (risk factors: healthcare exposures, instrumentation)
- Enterococci
- MSSA, MRSA
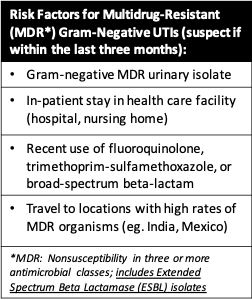
- Multi-drug resistant (MDR) organism risk factors:
- E. coli most common, then:
Adapted from UpToDate: Acute simple cystitis in women.
-
- Note: E. coli sequence type 131 (ST 131) is a major global cause of UTIs with ESBL and fluoroquinolone resistance.
Recommended Treatment
Management Considerations1:
- Empiric antibiotics:
- Initiate early.
- Use regional/institutional antibiogram (use Spectrum App) to help with antibiotic selection.
- Consult urology if anatomic/functional abnormalities.
- Consider obtaining appropriate imaging.
Empiric Antibiotics1:
- Obtain urine gram stain, culture and sensitivity.
- Inpatient:
- If critically ill, not improving on antibiotics, or urinary tract obstruction:
- Cover for ESBL organisms and Pseudomonas
- Antipseudomonal carbapenems
- Imipenem 500 mg IV q 6h
- Meropenem 1 gram IV q 8h
- Antipseudomonal carbapenems
- Cover for MRSA
- Vancomycin:
- 15 mg/kg/dose (usual maximum: 2 g/dose initially) q 12h is a usual starting dose in most nonobese patients with normal renal function.
- Alternatives:
- Daptomycin**
- 6 mg/kg IV q 24h
- Linezolid**
- 600 mg IV q 12h
- **Note: Daptomycin or Linezolid can also cover for vancomycin-resistant Enterococcus (VRE).
- Vancomycin:
- Obtain ID consult if carbapenem resistance from prior susceptibility result.
- Note: In communities where prevalence of MDR is low, narrower spectrum regimens are an option.
- Cover for ESBL organisms and Pseudomonas
- Non-critically ill or no concern for urinary tract obstruction:
- No risk factors for MDR gram negative organism:
- Treatment options:
- Ceftriaxone 1 gram IV q 24h
- Piperacillin-tazobactam 3.375 grams IV q 6h
- Oral or parenteral fluoroquinolones (ciprofloxacin or levofloxacin).
- No previous cultures resistant to fluoroquinolones in last three months.
- Community prevalence of E. coli fluoroquinolone resistance 10%.
- Options:
- Ciprofloxacin
- Oral: 500 mg q 12h x 5-7 days
- Oral: extended release: 1,000 mg q 24 h x 5-7 days
- IV: 400 mg q 12h x 5-7 days
- Levofloxacin
- Oral: 750 mg q 24h x 5-7 d
- IV: 750 mg q 24h x 5-7 d
- Ciprofloxacin
- If suspect Enterococcus or Staphyloccus, piperacillin-tazobactam preferred.
- If concern for Pseudomonas aeruginosa, use either:
- Piperacillin-tazobactam 4.5 grams IV q 6h (note: this is a higher dose than indicated above)
- Fluoroquinolone
- Cefepime 2 grams IV q 8h
- Ceftazidime 2 grams IV q 8h
- If concern for drug resistant gram positive, add either:
- Vancomycin 15 mg/kg IV q 12h for MRSA
- Linezolid 600 mg IV or PO q 12h for VRE
- Daptomycin 6 mg/kg IV q 24h for VRE
- Treatment options:
- No risk factors for MDR gram negative organism:
- 1 or more risk factors for MDR gram negative organism:
- Cover for ESBL organisms and Pseudomonas
- Antipseudomonal carbapenem
- Imipenem 500 mg IV q 6h
- Meropenem 1 gram IV q 8h
- Antipseudomonal carbapenem
- If Enterococcus species or MRSA are suspected, add either:
- Vancomycin 15 mg/kg IV q 12h for MRSA
- Linezolid 600 mg IV or PO q 12h for VRE
- Daptomycin 6 mg/kg IV q 24h for VRE
- Cover for ESBL organisms and Pseudomonas
- If critically ill, not improving on antibiotics, or urinary tract obstruction:
- Outpatient:
- For non-critically ill patients appropriate for outpatient management with oral antibiotics.
- Low risk for MDR infection:
- Fluoroquinolones (PO):
- Ciprofloxacin 500 mg q 12h x 5-7d
- Ciprofloxacin 1000 mg extended release q 24h x 5-7d
- Levofloxacin 750 mg q 24h x 5-7d
- Note:If E.coli resistance >10%, use single dose IV/IM prior to oral fluoroquinolone.
- IV/IM options:
- Ceftriaxone 1 gram (first line)
- Ertapenem 1 gram (second line)
- Gentamicin or tobramycin 5 mg/kg (if either above choices cannot be used)
- Oral options:
- Ciprofloxacin 500 mg q 12h x 5-7d
- Ciprofloxacin 1000 mg extended release q 24h x 5-7d
- Levofloxacin 750 mg q 24h x 5-7d
- IV/IM options:
- Non-fluoroquinolones:
- For patients with contraindications to fluoroquinolones
- Single dose IV/IM followed by oral non-fluoroquinolone:
- IV/IM options:
- Ceftriaxone 1 gram (first line)
- Ertapenem 1 gram (second line)
- Gentamicin or tobramycin5 mg/kg (if either above choices cannot be used)
- Oral options:
- Trimethoprim-sulfamethoxazole double-strength (160 mg/800 mg) tablet BID x 7-10 days
- Amoxicillin-clavulanate 875 mg BID x 10-14 days
- Cefadroxil 1 g BID x 10-14 days
- IV/IM options:
- Note: If concerned for more serious infection, continue IV antibiotics until urine culture and susceptibility results
- Fluoroquinolones (PO):
- High risk MDR infection:
- Initial dose of ertapenem 1 gram IV/IM, followed by fluoroquinolone (if no contraindications, no prior 3 month history of use or documented resistance). See above PO doses.
- If unable to take fluoroquinolones, recommend continuing ertapenem 1 gram IV/IM once daily, until urine culture and susceptibility results.
- Low risk for MDR infection:
- For non-critically ill patients appropriate for outpatient management with oral antibiotics.
Directed Antibiotic Therapy1:
-
- Use results of urine culture susceptibility to narrow empiric antibiotics, if possible.
- Switch from IV to PO with symptom improvement.
- In Complicated UTI, acceptable PO antibiotics include:
- levofloxacin 750 mg once daily x 5-7d
- ciprofloxacin 500 mg BID x 5-7d
- ciprofloxacin 1000 extended release once daily x 5-7d
- trimethoprim-sulfamethoxazole one double-strength (160 mg/800 mg) tablet BID x 7-10d
- Consider oral beta-lactams if there is susceptibility and other antibiotics not feasible.
- *Use of nitrofurantoinand fosfomycin should be avoided – do not achieve appropriate tissue concentrations outside of bladder.
- *Duration of antibiotics should also be dependent on clinical response.
- In Complicated UTI, acceptable PO antibiotics include:
Criteria For Hospital Admission
- Sepsis
- Unable to tolerate oral medications or oral hydration
- Suspected urinary tract obstruction
- Poor patient adherence
Criteria for Outpatient Management
- Able to stabilize patient with rehydration and antibiotics in ED
- Able to discharge home on oral antibiotics
- Patient able to have close follow-up
Follow Up
- Patient’s treated in outpatient setting need follow up in 24H
- If no improvement or worsening symptoms or if recurrent symptoms after treatment1
- Abdo/Pelvis imaging (generally with CT)
- Repeat urine culture and susceptibility
- If other causative organisms found, tailor treatment to that
- Note1,3: if hematuria on initial presentation, repeat urinalysis in approximately 6 weeks following completion of antibiotics to evaluate for persistent hematuria
Quality Of Evidence?

High
We are highly confident that the true effect lies close to that of the estimate of the effect. There is a wide range of studies included in the analyses with no major limitations, there is little variation between studies, and the summary estimate has a narrow confidence interval.
Moderate
We consider that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. There are only a few studies and some have limitations but not major flaws, there are some variations between studies, or the confidence interval of the summary estimate is wide.
Low
When the true effect may be substantially different from the estimate of the effect. The studies have major flaws, there is important variations between studies, of the confidence interval of the summary estimate is very wide.
Justification
The above summary is taken from recent literature reviews on emergency department diagnosis and management of UTI’s.
Related Information
Reference List
Relevant Resources
RESOURCE AUTHOR(S)

DISCLAIMER
The purpose of this document is to provide health care professionals with key facts and recommendations for the diagnosis and treatment of patients in the emergency department. This summary was produced by Emergency Care BC (formerly the BC Emergency Medicine Network) and uses the best available knowledge at the time of publication. However, healthcare professionals should continue to use their own judgment and take into consideration context, resources and other relevant factors. Emergency Care BC is not liable for any damages, claims, liabilities, costs or obligations arising from the use of this document including loss or damages arising from any claims made by a third party. Emergency Care BC also assumes no responsibility or liability for changes made to this document without its consent.
Last Updated Jun 10, 2020
Visit our website at https://emergencycarebc.ca
COMMENTS (0)
Add public comment…


POST COMMENT
We welcome your contribution! If you are a member, log in here. If not, you can still submit a comment but we just need some information.