HIV Post-Exposure Prophylaxis (PEP)
Cardinal Presentations / Presenting Problems, Infections, Special Populations
Context
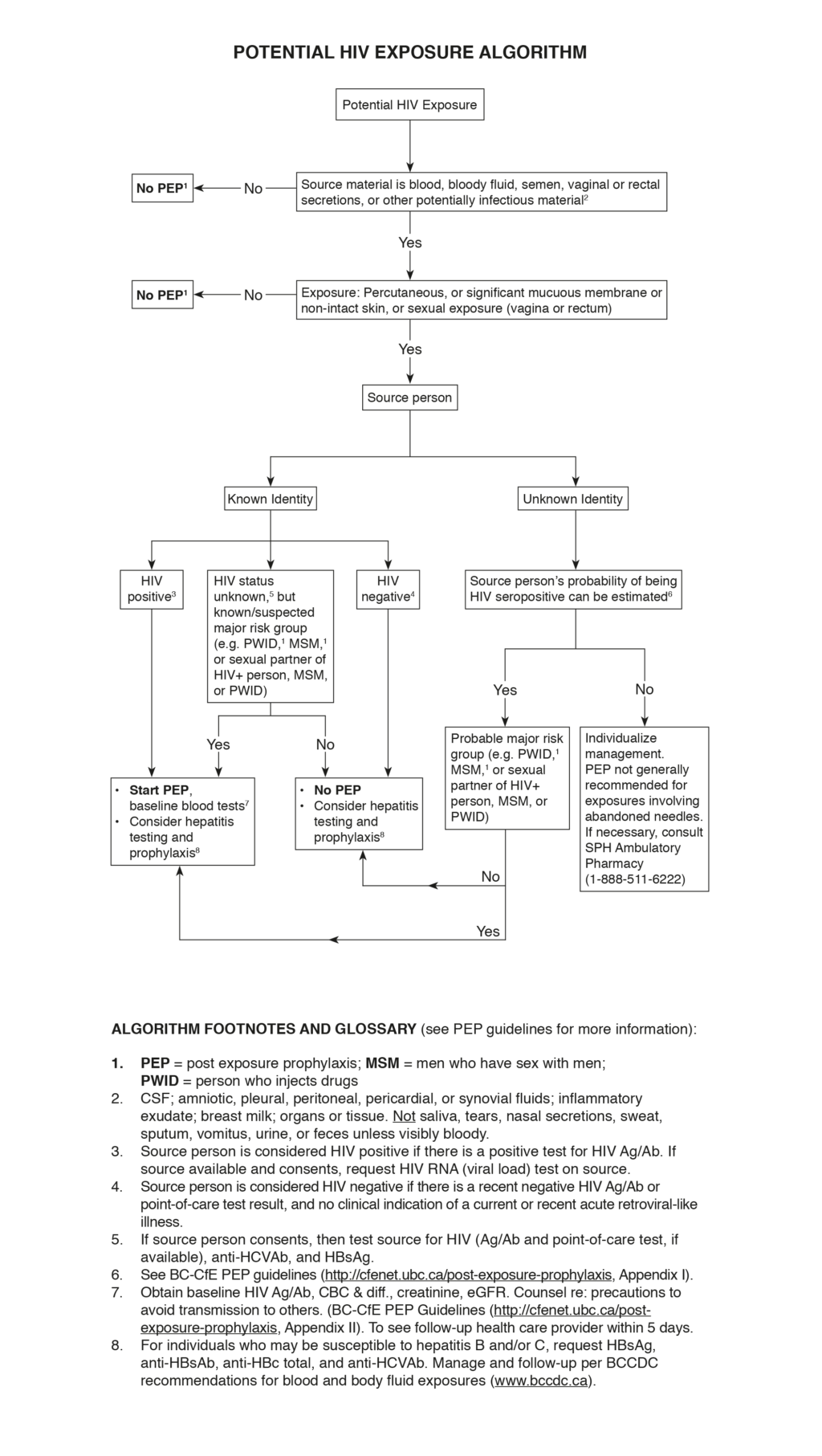
- Patient population: Persons who have recently experienced potential exposure to HIV as a result of an accidental event in a workplace, community setting, sexual assault, or consensual sexual or needle-sharing activity.
- Why is this important: The likelihood of HIV transmission can be significantly reduced (>80%) by provision of antiretrovirals for post-exposure prophylaxis (PEP) if started as soon as possible, preferably within 2 hours (and not more than 72 hours) after the event.
- PEP is indicated only when there is a significant risk of HIV transmission from the exposure, specifically when all of the following apply:
- Exposed person is not HIV positive
- Exposure is to blood or a potentially infectious body fluid (semen, vaginal secretions, or other body fluid visibly contaminated with blood). Saliva does not transmit HIV unless visibly bloody.
- Significant exposure via percutaneous route (needlestick or cut where the injury bleeds), mucous membrane (eye, mouth, nose, vagina, anorectal), or non-intact skin (healing wound <3 days old or skin lesion causing significant disruption of the epidermis)
- Source is HIV positive or at high risk for HIV infection (e.g. men who have sex with men (MSM), people who inject drugs (PWID))
- PEP is not recommended for needle-sticks from an abandoned or discarded needle outside a health care or high-risk setting (e.g. supervised injection site). For further info see BC-Centre for Excellence in HIV/AIDS Post-Exposure Prophylaxis (PEP) Guidelines (Section 2.4.3).
Diagnostic Process
Potential HIV Exposure Algorithm (figure)
- Assess the exposed person:
- Draw blood for HIV Ag/Ab test (or do point-of-care test, if available) at baseline in all cases, to rule out pre-existing HIV infection.
- If exposed person belongs to a group at high risk for HIV infection (e.g. MSM, PWID), assess history of high-risk activities (e.g. condomless sex, needle sharing) within the previous month and symptoms of acute HIV infection within previous 6 weeks; the HIV test may be unreliable during acute infection (seroconversion window period).
- Draw blood for serum creatinine and estimated glomerular filtration rate (eGFR).
- If the exposed person is female, determine whether she is pregnant; if uncertain, do pregnancy test.
- Assess the exposure event to determine potential for HIV transmission:
- Time since event: PEP is only effective if started within 72 hours (preferably as soon as possible).
- Material to which exposure has occurred: blood or other body fluid; quantity
- Type/route of exposure: percutaneous (hollow bore needle [gauge size] or solid instrument; depth and extent of cut or puncture); mucous membrane; skin (whether intact)
- If sexual exposure, assess whether consensual or assault.
- Use of protection (e.g. gloves, condom, eye protection) and whether intact at time of event.
- Assess the source person to determine likelihood of being HIV positive:
- If source is known, determine HIV status or whether belonging to a group at high risk for HIV infection (e.g. MSM, PWID).
- If source is unknown, estimate likelihood of being HIV positive or belonging to a group at high risk for HIV infection (e.g. MSM, PWID).
Note: For accidental needlesticks, can also use the HIV Needle Stick Risk Assessment Stratification Protocol (RASP)
- If uncertain whether PEP is indicated, contact the BC Centre for Excellence in HIV/AIDS (St. Paul’s Hospital Ambulatory Pharmacy) (1-888-511-6222).
Consider risk of hepatitis B, hepatitis C, sexually transmitted infections, and pregnancy.
Recommended Treatment
- If significant risk of HIV exposure has occurred within the previous 72 hours, provide the 5-day PEP starter kit (available in ER). Patients should be instructed to take the first dose of all three medications together as soon as possible:
- Tenofovir DF one tablet (300 mg) once a day
- Lamivudine one tablet (150 mg) twice a day
- Raltegravir one tablet (400 mg) twice a day
- Do not delay starting PEP while waiting for results of lab tests.
- Send home with remainder of 5-day PEP starter kit.
- Patients and providers can contact the BC Centre for Excellence in HIV/AIDS (St. Paul’s Hospital Ambulatory Pharmacy) (1-888-511-6222) for any questions.
Criteria For Safe Discharge Home
- Follow-up information for the patient is provided in the 5-day PEP starter kit.
- Instruct patient to follow up with primary care provider who will consult the BC Centre for Excellence in HIV/AIDS (BC-CfE) to evaluate need for full 28-day course of PEP, before finishing the 5-day kit.
- Patients seen at St. Paul’s Hospital ED who receive PEP following a consensual exposure should contact St. Paul’s Hospital IDC Clinic (604-806-8060) before finishing the 5-day kit.
- For questions or concerns, patients can call the BC Centre for Excellence in HIV/AIDS (St. Paul’s Hospital Ambulatory Pharmacy) at 1-888-511-6222.
Quality Of Evidence?

High
We are highly confident that the true effect lies close to that of the estimate of the effect. There is a wide range of studies included in the analyses with no major limitations, there is little variation between studies, and the summary estimate has a narrow confidence interval.
Moderate
We consider that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. There are only a few studies and some have limitations but not major flaws, there are some variations between studies, or the confidence interval of the summary estimate is wide.
Low
When the true effect may be substantially different from the estimate of the effect. The studies have major flaws, there is important variations between studies, of the confidence interval of the summary estimate is very wide.
Justification
The quality of evidence for PEP is moderate for occupational needlestick exposures and low for sexual exposures, being based mainly on primate studies and observational studies in humans. The very low risk of HIV transmission from a single exposure and ethical constraints preclude the possibility of obtaining higher quality data.
Related Information
OTHER RELEVANT INFORMATION
BC-Centre for Excellence in HIV/AIDS Post-Exposure Prophylaxis (PEP) Guidelines
BC Centre for Disease Control, Blood and Body Fluid Exposure Management
HIV Needle Stick Risk Assessment Stratification Protocol (RASP)
Sexual Assault Service Resources, BC Women’s Hospital and Health Centre
CME/CPD Training: HIV Prevention Online Course, BC Centre for Excellence in HIV/AIDS Education & Training
Reference List
Relevant Resources
RELEVANT RESEARCH IN BC
Sepsis and Soft Tissue InfectionsRESOURCE AUTHOR(S)

DISCLAIMER
The purpose of this document is to provide health care professionals with key facts and recommendations for the diagnosis and treatment of patients in the emergency department. This summary was produced by Emergency Care BC (formerly the BC Emergency Medicine Network) and uses the best available knowledge at the time of publication. However, healthcare professionals should continue to use their own judgment and take into consideration context, resources and other relevant factors. Emergency Care BC is not liable for any damages, claims, liabilities, costs or obligations arising from the use of this document including loss or damages arising from any claims made by a third party. Emergency Care BC also assumes no responsibility or liability for changes made to this document without its consent.
Last Updated Aug 22, 2018
Visit our website at https://emergencycarebc.ca
COMMENTS (1)
Add public comment…


POST COMMENT
We welcome your contribution! If you are a member, log in here. If not, you can still submit a comment but we just need some information.