Febrile Neutropenia in Adults
Cardinal Presentations / Presenting Problems, Hematological / Oncological, Infections
Context
- Neutropenia can result from decreased production (e.g., drug-induced, infection, malignancy, nutrition deficiency), redistribution (e.g., margination to the spleen), or immune destruction (e.g., autoimmune conditions).
- Due to a muted immune response, fever may be the principal and only sign of invasive infection in neutropenic patients.
- Prompt recognition of a neutropenic fever is prudent to ensure initiation of empiric antimicrobial therapy and to avoid progression to a sepsis syndrome.
Definitions
- Fever: A single oral temperature of ≥ 38.3° C (101° F) OR a temperature ≥ 38° C (100.4° F) which lasts more than 1h.
- Neutropenia: An abnormally low number of neutrophils in the blood (ANC < 1.0 x 109/L). The lower the neutrophil count, the greater the risk of infection.
- Absolute Neutrophil Count (ANC) Calculator https://www.mdcalc.com/calc/19/absolute-neutrophil-count-anc
Diagnostic Process
1. History
- A thorough history should be conducted with emphasis on new site-specific symptoms, recent antibiotic treatment, surgical history, underlying comorbid conditions, and past microbiology records (e., history of antibiotic resistant organisms or bacteremia).
2. Physical Examination
- Focus on identifying a focus of infection including, but not limited to: presence of indwelling IV catheters, skin lesions and lymph nodes, oropharynx, chest and lungs, abdomen, genital and perianal/rectal area*, central nervous system.
- * DRE should be avoided in neutropenic or immunocompromised patients.
3. Investigations
Routine Investigations
- CBC with differential
- Creatinine, electrolytes
- Liver function tests, coagulation screen
- CRP
- Blood cultures: two sets – one peripheral and one from CVC
- Microbiologic testing from suspected sites of infection: urinalysis and culture, sputum microscopy and culture, stool microscopy and culture, skin lesion (aspirate, biopsy, swab, culture), chest radiograph, lumbar puncture.
Further Testing
- High-resolution chest CT (if pyrexial despite 72h of appropriate antibiotics)
- Bronchoalveolar lavage
4. Severity Assessment
- A prognosis clinical prediction rule, in addition to clinical judgement, is recommended. Two are commonly used:
- Multinational Association for Supportive Care in Cancer (MASCC) score
- Clinical Index of Stable Febrile Neutropenia (CISNE) score
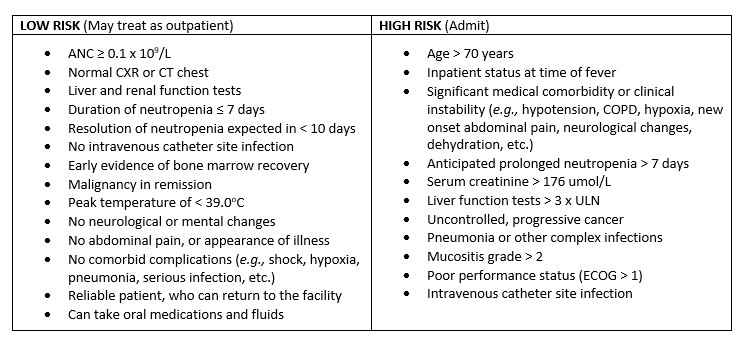
Management
EMPIRIC OUTPATIENT TREATMENT
Recommended Antibiotics
- Levofloxacin 750mg daily PO or Ciprofloxacin 750 mg PO Q12H AND Amoxicillin/Clavulanate 875/125 mg PO Q12H, or
- Levofloxacin 750mg daily PO or Ciprofloxacin 750 mg PO Q12H AND Clindamycin 600 mg PO Q8H if penicillin allergy.
- Fluoroquinolones are NOT recommended if significant patient exposure in the past 3 months.
- Antimicrobial therapy duration: continue until the infection has resolved AND the patient is no longer neutropenic.
Plan
- Formally re-evaluate patient in 2 to 3 days.
- If AFEBRILE for ≥ 48 hours AND neutrophils ≥ 2 consecutive days and increasing, no positive source of infection identified and patient clinically stable, may discontinue antibiotics and monitor patient.
- If FEBRILE, admit patient for further investigations and initiation of appropriate antimicrobial therapy.
EMPIRIC INPATIENT TREATMENT
Recommended Antibiotics
- Piperacillin-Tazobactam 4.5 g IV QID, or [1,2]
- Imipenem 500 mg IV Q6H or Meropenem 1g IV Q8H, or [1,2]
- Cefepime 2g IV Q8H or Ceftazidime 2g IV Q8H (NOT recommended as monotherapy in areas at risk for ESBL producing bacteria). [1,2]
- If anaphylaxis allergy to beta-lactams, treat with Vancomycin + Aminoglycoside + Ciprofloxacin.
- NOTE: avoid aminoglycosides or other nephrotoxic agents in patients receiving cisplatin or other nephrotoxic chemotherapy.
Adjunct Antibiotics
- If positive blood culture for gram-positive organism, catheter-related infection, skin or soft-tissue infection, known or suspected MRSA, or suspicion for Clostridium difficile, add:
- Vancomycin 25 mg/kg IV loading dose, followed by 15 mg/kg IV [3]
- If anaerobic infection (g., intra-abdominal) suspected, add:
- Metronidazole 500 mg IV Q12H
- If resistance suspected or there are complications (g., hypotension, persistent fever, pneumonia, etc.), add: [3]
- Tobramycin or Gentamicin 6-7 mg/kg IV Q24H, or
- Ciprofloxacin 400 mg IV Q8-12H, or
- If atypical pneumonia suspected (g., Legionella or Mycoplasma), add:
- Azithromycin 500 mg IV daily, or
- Moxifloxacin 400 mg IV daily or Levofloxacin 750 mg IV daily.
Antifungal therapy
- Consider in those with persistent fevers, despite receiving 3-5 days of broad-spectrum antibiotic therapy.
Related Information
OTHER RELEVANT INFORMATION
Empiric treatment for febrile neutropenia. (2015). BC Cancer Agency. Available at http://www.bccancer.bc.ca/Documents/BCCA%20Febrile%20Neutropenia%20Guidelines.pdf.
Reference List
Freifeld, A. G., Bow, E. J., Sepkowitz, K. A., Boeckh, M. J., Ito, J. I., Mullen, C. A., … & Wingard, J. R. (2011). Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clinical infectious diseases, 52(4), e56-e93.
Flowers, C. R., Seidenfeld, J., Bow, E. J., Karten, C., Gleason, C., Hawley, D. K., … & Ramsey, S. D. (2013). Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol, 31(6), 794-810.
Klastersky, J., De Naurois, J., Rolston, K., Rapoport, B., Maschmeyer, G., Aapro, M., & Herrstedt, J. (2016). Management of febrile neutropaenia: ESMO clinical practice guidelines. Annals of Oncology, 27, v111-v118.
Taplitz, R. A., Kennedy, E. B., Bow, E. J., Crews, J., Gleason, C., Hawley, D. K., … & Flowers, C. R. (2018). Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J Clin Oncol, 36(14), 1443-1453.
RESOURCE AUTHOR(S)

DISCLAIMER
The purpose of this document is to provide health care professionals with key facts and recommendations for the diagnosis and treatment of patients in the emergency department. This summary was produced by Emergency Care BC (formerly the BC Emergency Medicine Network) and uses the best available knowledge at the time of publication. However, healthcare professionals should continue to use their own judgment and take into consideration context, resources and other relevant factors. Emergency Care BC is not liable for any damages, claims, liabilities, costs or obligations arising from the use of this document including loss or damages arising from any claims made by a third party. Emergency Care BC also assumes no responsibility or liability for changes made to this document without its consent.
Last Updated Feb 08, 2023
Visit our website at https://emergencycarebc.ca
COMMENTS (0)
Add public comment…


POST COMMENT
We welcome your contribution! If you are a member, log in here. If not, you can still submit a comment but we just need some information.